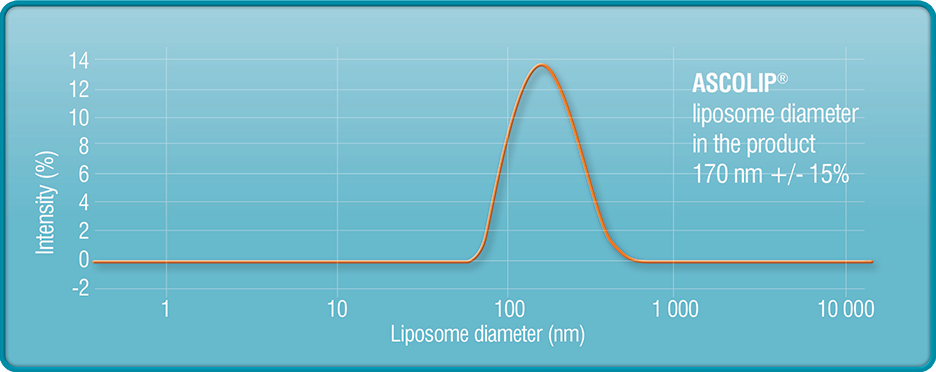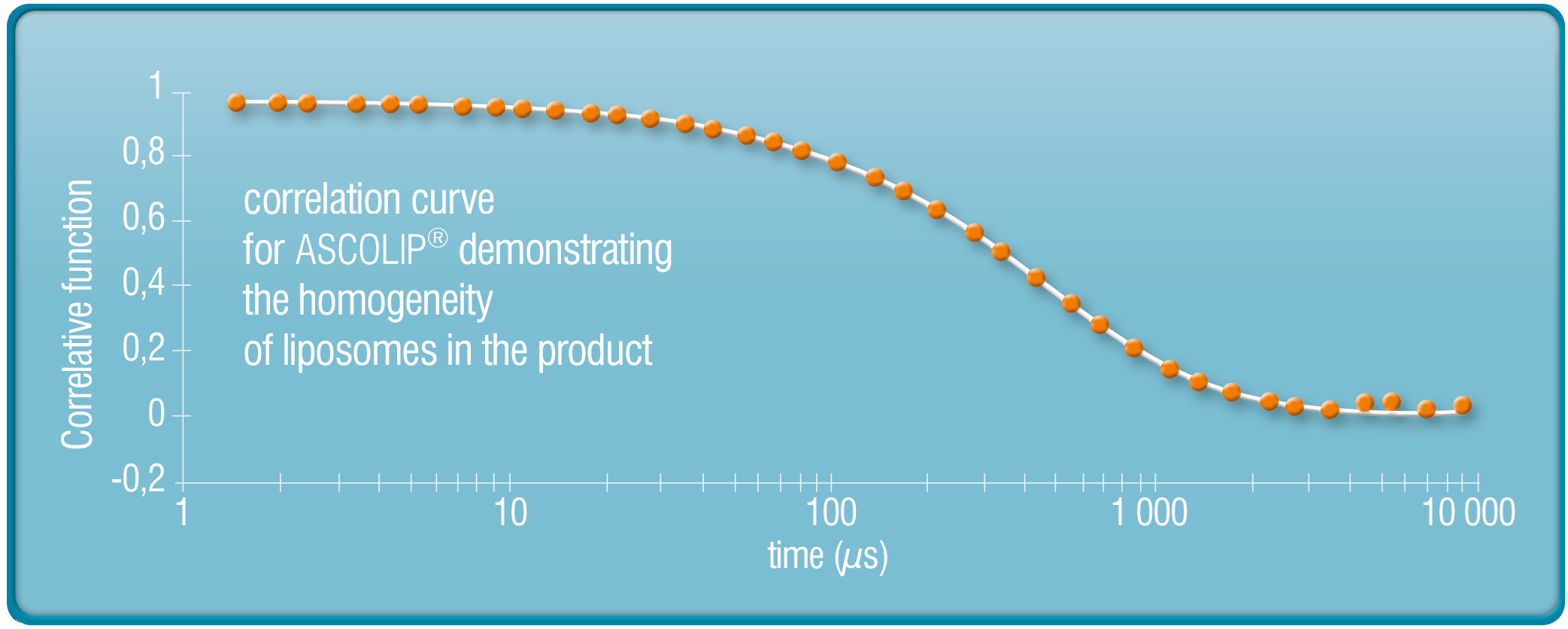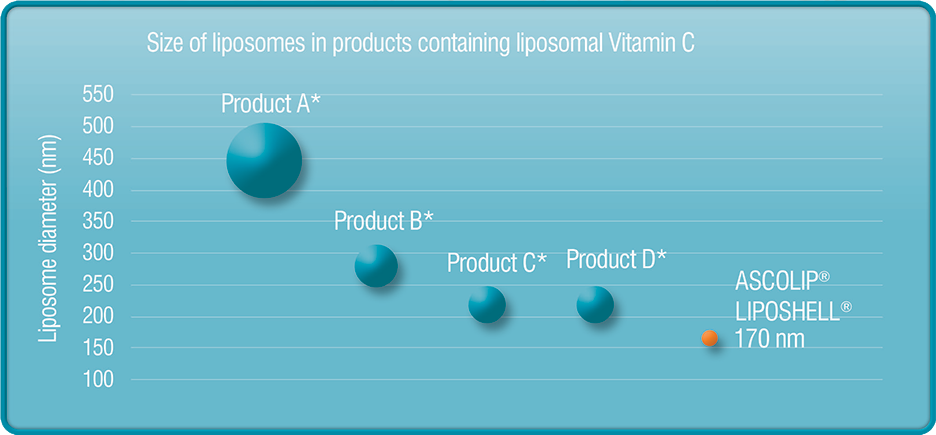
STUDIES OF QUALITY OF LIPOSHELLВ® TECHNOLOGY PRODUCTS
LIPOSHELLВ®* technology products are manufactured in compliance with the highest quality standards, using innovative manufacturing processes which give them globally unique qualities.
Quality of our products is subjected to regular controls both during the manufacturing process and in independent microbiology laboratories.
ASCOLIPВ® meets both PN-EN 7218:2008/A1:2013-10 standards and the Pharmacopoeia, edition X, as appropriate for oral formulations. Meeting the above requirements ensures proper quality of pharmaceutical raw materials, foodstuffs and medicinal products, which in turn guarantees quality and safety of their use.
*LIPOSHELLВ® - the patented technology- international application no PCT/EP2018/057400